The Spinach Chromatography Lab:
Our question: Do green leaves contain other pigments?
Materials:
- . Obtain a strip of chromatography paper.
- Use a ruler to measure and draw a light pencil line 2-cm above the bottom of the paper strip.
- Here is the tricky part! Place the edge of the spinach leaf over the pencil line and using the edge of a coin gently press on the spinach leaf to create a single green line over the pencil line. You want this line to be thin and concentrated with the pigment from the spinach leaf. Therefore, repeat this edging process carefully about 3-4 times. Be sure not to press too hard or you will poke a hole through the paper.
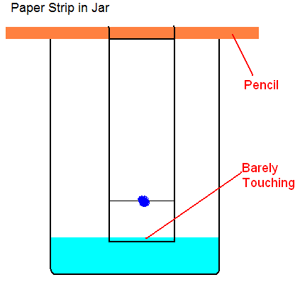
- Tape the top of the paper strip to a pencil so that the end of the strip with the green line hangs down. The pencil should be able to sit across the top of the beaker with the bottom of the paper strip just touching the bottom of the beaker. Cut off any excess paper from the TOP of the strip if it is too long.
- Remove the pencil/paper strip contraption from the beaker for the moment. Record observations in data table.
- Carefully add isopropyl alcohol to the beaker until it reaches a depth of 1-cm in the beaker.
- Lay the pencil across the top of the beaker with the paper strip extending into the alcohol. MAKE SURE THAT THE LEVEL OF THE ALCOHOL IS BELOW THE GREEN LINE ON YOUR PAPER STRIP! IF THE ALCOHOL IS GOING TO COVER THE GREEN LINE, POUR OUT SOME ALCOHOL BEFORE YOU GET THE GREEN LINE WET!
- Observe as the alcohol gets absorbed and travels up the paper by capillary action. This may take up to 20 minutes. Do not touch your experiment during this time.
- When the alcohol has absorbed to approximately 1-cm below the pencil, you may remove the pencil/paper strip from the beaker to dry on your counter. With a pencil, mark the distance the alcohol has traveled on the paper, as well as the distance each pigment has traveled.
- Using colored pencils, draw your results in the data table.
- Using a ruler and the following formula, measure the Rf values of each pigment.
- Since the fastest molecules will travel the greatest distance, or to the highest point along the strip,
- the relative distances can be measured, and the flow rate (migration) of the molecules (Rf) can be
- calculated by using the following formula:
- Rf = Distance pigment traveled
- Distance solvent traveled


No comments:
Post a Comment